’Restriction point control’
emlõs sejtekben
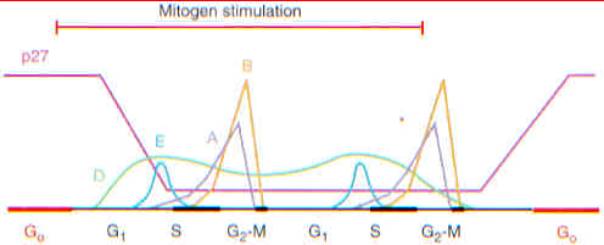
Fluctuations of cyclins and p27 sup KIP1 during the cell cycle.
Expression of cyclins E, A, and B (mitotic cyclin) is periodic. D-type cyclins
are expressed throughout the cycle in response to mitogen stimulation (the
period indicated by the top bar), and a less idealized scheme would indicate
that different ones (D1, D2, and D3) are induced by various signals in a cell
lineage-specific manner. The cyclins assemble with more stably expressed CDKs
to temporally regulate their activities. D-type cyclins form complexes with
CDK4 and CDK6; cyclin E with CDK2; cyclin A with CDK2 (in S phase) and with
CDC2 (CDK1) (in late S and G sub 2); and cyclin B with CDC2. The holoenzymes
can be negatively regulated by phosphorylation, so that even though cyclin
B-CDC2 complexes progressively assemble as B cyclins accumulate, their
catalytic activity is restricted to mitosis. p27 levels are high in quiescent
cells, fall in response to mitogenic stimulation, remain at lower threshold
levels in proliferating cells, and increase again when mitogens are withdrawn.
In proliferating cells, most p27 is complexed with cyclin D-CDK complexes.
From: Sherr: Science, Volume 274(5293).December 6, 1996.pp 1672-1677.
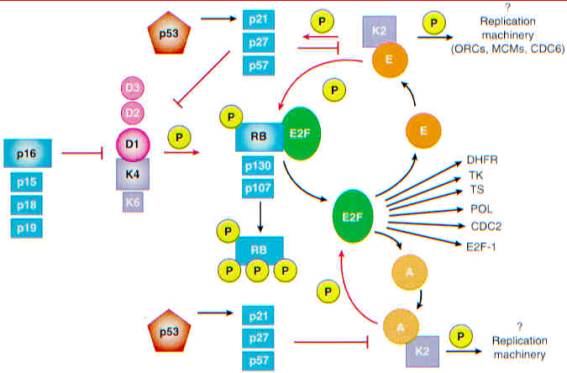
Restriction point control. RB phosphorylation
triggered by cyclin D-dependent kinases releases RB-bound E2F. Rather than
illustrating the many E2F-DP heterodimers that are differentially regulated by
various RB family members, E2F "activity" is shown for simplicity.
E2F triggers the expression of dihydrofolate reductase (DHFR), thymidine kinase
(TK), thymidylate synthase (TS), DNA polymerase-alpha (POL), CDC2, cyclin E and
possibly cyclin A, and E2F-1 itself. This establishes a positive feedback loop
promoting RB phosphorylation by cyclin E-CDK2, contributing to the irreversibility
of the restriction point transition and ultimately making it
mitogen-independent. In parallel, cyclin E-CDK2 may oppose the inhibitory
action of p27 sup KIP1 by phosphorylating it. This allows cyclin A-CDK2 and
possibly cyclin E-CDK2 to start S phase. Possible CDK substrates include those
of the origin-recognition complex (ORC), minichromosome maintenance proteins
(MCMs), and CDC6, all of which assemble into preinitiation complexes. Once
cells enter S phase, cyclin A-CDK2 phosphorylates DP-1 and inhibits E2F binding
to DNA. Like p27, p53-inducible p21 sup CIP1 can induce G sub 1 arrest by
inhibiting the cyclin D-, E-, and A-dependent kinases. In contrast, INK4
proteins antagonize only the cyclin D-dependent kinases. The proteins most
frequently targeted in human cancers are highlighted. Arrows depicting
inhibitory phosphorylations (P) or inactivating steps are shown in red, and
those depicting activating steps are shown in black.