myc-src.htm
Src
kináz-család, kilenc tag. A humán daganatokban az src szerepel
SH3 és SH2 domainek
moduláló domainek
intra- és intermolekuláris kapcsolatok fehérjékkel
befolyásolják a
src katalítikus aktivitását
SH1 kináz domain
tirozin kináz aktivitás
szubsztrát
kötés
a
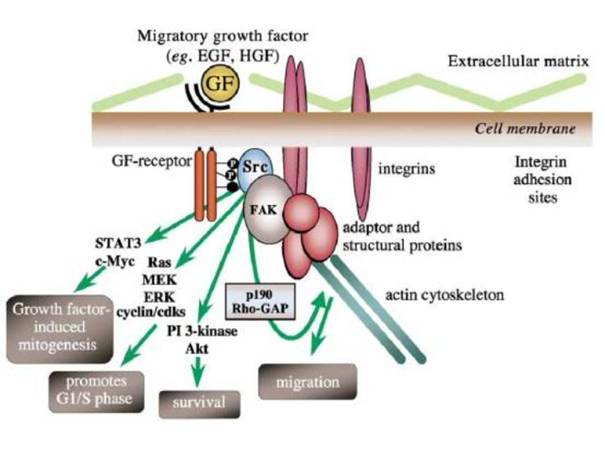
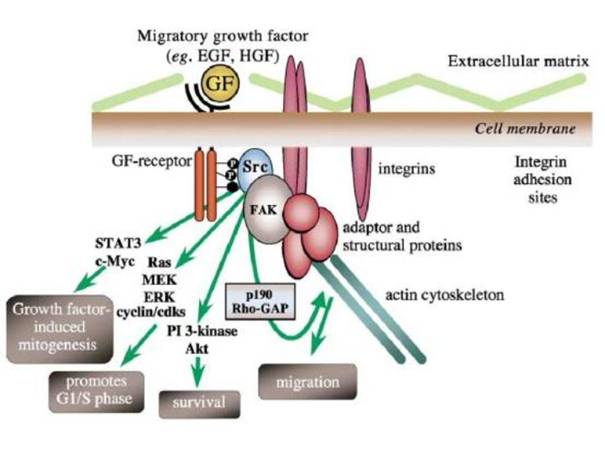
src szerepe:
Integrin-dependens adhézió → Src-mediált FAK (Y925) foszforiláció
kötőhelyhez a Grb2 csatlakozik
→ Ras-MEK(MAPK)-Erk szignálút
FAK-independens
módon is kialakulhat proliferatív válasz:
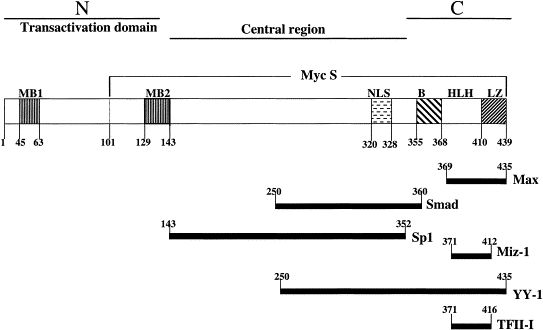
a c-myc fehérje fontos domainjei: protein-protein
kötés
|
|
|
The central region and the C-terminal domain of c-Myc are responsible for protein–protein interactions that lead to transcriptional repression by c-Myc. The c-Myc protein consists of three domains: N-terminal, central, and C-terminal. The C-terminal part of c-Myc contains a basic helix–loop–helix–leucine zipper motif (bHLH-LZ) that is necessary for interaction with Max and other proteins. Factors that are involved in Inr-dependent transcriptional repression such as Miz-1, YY-1, and TFII bind to the C-terminal domain of c-Myc. Transcription factors Sp1 and Smad bind to the central region of the c-Myc protein. The c-Myc protein amino acids involved in interactions with other proteins are indicated. |
myc-repression
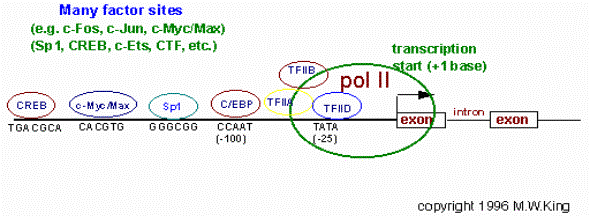
CAGT motif
(szekvenciamotívum), az egyik iniciátor (Inr)
motívum
|
|
|
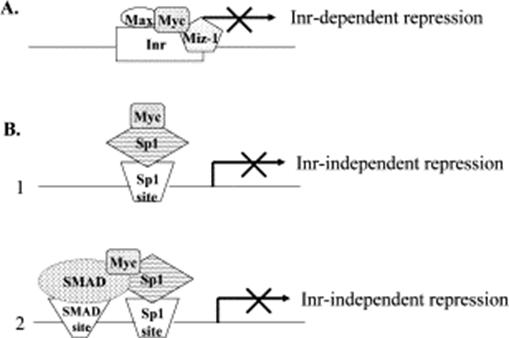
Mechanisms of transcriptional repression by c-Myc.
(A) c-Myc represses transcription of some target genes by Inr-dependent mechanism. Myc–Max heterodimers bind to the Inr element in target gene promoters and associate with Miz-1 or other transcriptional activators, thus interfering with their activities. (B) c-Myc represses target genes transcription by Sp1-dependent mechanism. c-Myc interacts with the Sp1 transcription factor (1) or with the Smad–Sp1 complex (2) via the c-Myc central region and inhibits Sp1 transcriptional activity. This mechanism does not require DNA binding or interaction with the c-Myc partner Max. |
ugyanis: sok promóter
többféle TF kötőhelyet tartalmaz